Additional information
| Active substance | Aripiprazole |
|---|---|
| Active Half-Life | 24 hours |
| Classification | Antipsychotic |
| Dosage | 5-30 mg/day |
| Acne | No |
| Water Retention | No |
| HBR | Perhaps |
| Hepatotoxicity | Low |
| Aromatization | No |
| Lab Test | Monitoring blood levels is not typically required, but lipid profiles, blood glucose, and weight should be monitored |
| Also known as | OPC-14597, OPC-31 |
| WAREHOUSE | International Warehouse 2 |
| Blood pressure | Can cause orthostatic hypotension |
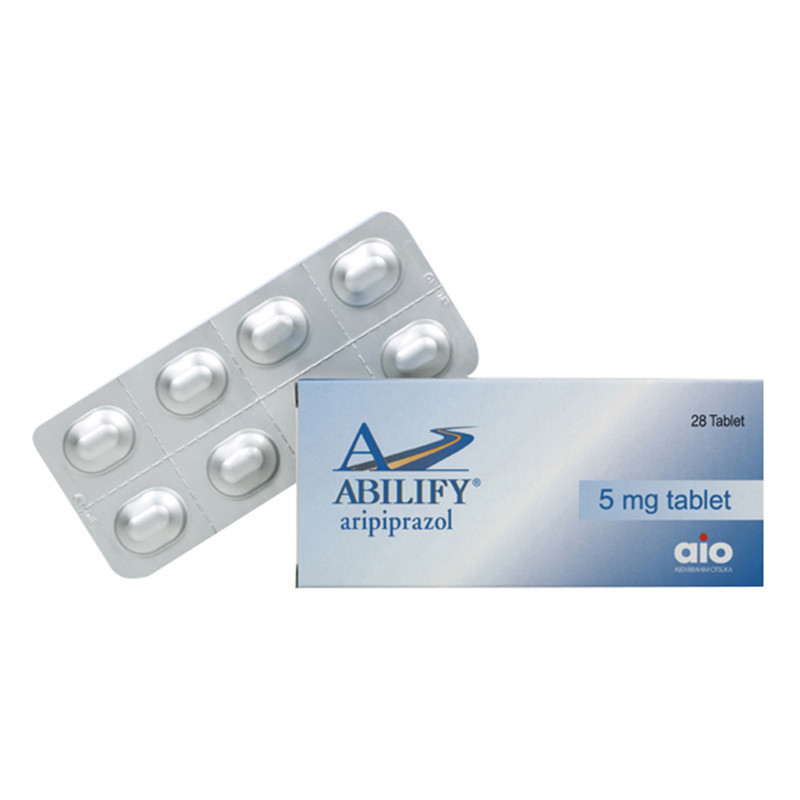
| Trade name | Abilify, Aripiprex |
| Storage conditions | Store at room temperature, 15-30В°C (59-86В°F) |
| Chemical name | 7-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one |
| Formula | C23H27Cl2N3O2 |
| Substance class | Atypical antipsychotic |
| Main action | Dopamine D2 and serotonin 5-HT1A receptor partial agonist |
| Half-life | Approximately 75 hours |
| Dosage (medical) | Typically starts at 10-15 mg once daily for schizophrenia, 2-15 mg once daily for bipolar disorder |
| Dosage (sports) | Not applicable |
| Effects | Antipsychotic, mood stabilization, reducing symptoms of schizophrenia and bipolar disorder |
| Side effects | Anxiety, insomnia, nausea, vomiting, blurred vision, somnolence, restlessness, tremor |
| Use in sports | None |
| Manufacturer | Abdi Ibrahim |


Reviews
There are no reviews yet.